Immunotherapy is increasingly used to treat cancers, as it has numerous benefits for patients. This treatment is characterized by high efficacy and prolonged life expectancy, through the maintenance of the immune system response over time. Much promising research is being conducted on the subject. However, immunotherapy has one major limitation. It remains mostly ineffective in the treatment of pediatric cancers. Indeed, this therapeutic approach is based on a key concept: tumor-specific genetic mutations can be identified as non-self by the immune system. However, in childhood cancers, these mutations are few in number.
A study conducted by the team of Dr. Olivier Delattre, Inserm research director at the head of the Cancer, Heterogeneity, Instability and Plasticity unit (CHIP – Institut Curie/Inserm/University of Paris), has highlighted the expression of highly specific genes in Ewing’s sarcoma[1], and more broadly in certain sarcomas and pediatric tumors.
These results were published on May 11, 2022, in the journal Molecular Cell, with the support of the Ligue nationale contre le cancer, L’Etoile de Martin, la Course de l’Espoir, M la vie avec Lisa, ADAM, Couleur Jade, Dans les pas du Géant, Courir pour Mathieu, Marabout de Ficelle, Olivier Chape, Les Bagouzamanon, Enfants et Santé, Les Amis de Claire, Un Elan pour Lucas.
Genetic alteration characteristic of sarcoma shows novel activity
Many cancers are characterized by specific genetic mutations called “gene fusions” that result in the expression of oncogenic proteins (transcription factors). About 95% of Ewing’s tumors are due to a characteristic gene fusion: most often, it is a translocation[2] that occurs between chromosomes 11 and 22 and results in the synthesis of an abnormal protein, the transcription factor EWS-FLI-1. Fusions between two genes are found in a large number of sarcomas, resulting in the presence of these particular “chimeric[3] oncogenic” transcription factors.
These initial discoveries were made by the Diversity and Plasticity of Childhood Tumors team led by Dr. Delattre at the Institut Curie, which has taken another step forward in understanding the crucial role of EWS-FLI-1. The researchers reveal that this Ewing’s sarcoma-specific transcription factor induces the expression of a set of genes in regions of the genome that are normally “silent”, i.e. not transcribed. Their results indicate that these “neogenes” can be translated into highly specific peptides because they are highly expressed in Ewing’s sarcoma cells, whereas they are absent in normal cells of the body. The role of these transcription factors in metastatic processes is already known but this is the first time that such activity has been observed.
“The existence of these particular genetic mutations is found in many pediatric cancers, suggesting the possibility of immunotherapies targeting these tumor-specific proteins. This discovery could prove revolutionary for the management of childhood tumors, which are currently the 2nd leading cause of death in children under 15.” explains Dr. Delattre.
“We now need to demonstrate that these newly identified proteins can constitute real therapeutic targets for the development of immunotherapies. This is the subject of the research we are currently conducting in collaboration with the Immunity and Cancer Unit (Institut Curie/Inserm/University of Paris) headed by Ana-Maria Lennon and the Clinical Immunology Laboratory headed by Dr. Olivier Lantz at Institut Curie,” he concludes.
More generally, the study authors showed that hundreds of these neogenes can be detected in a variety of cancers characterized by oncogenic chimeric transcription factors. The high specificity and recurrent expression of these peptides in a wide variety of childhood sarcomas make them promising therapeutic targets for the development of immunotherapies for the treatment of pediatric cancers.
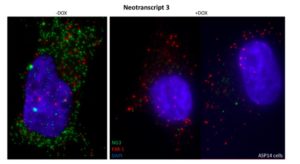
In the presence of the transcription factor EWSR1-FLI-1 (left), NG3 gene transcripts (green) – specific to Ewing’s sarcoma – are abundant. When EWSR1-FLI-1 is decreased (right), NG3 transcripts disappear completely. The FXR1 gene transcripts (red) – not dependent on EWSR1-FLI-1 – remain present.
@ Kyra Bergman, Antoine Coulon
Read the publication
Oncogenic chimeric transcription factors drive tumor-specific transcription, processing, and translation of silent genomic regions. Julien Vibert, Olivier Saulnier, Céline Collin, Floriane Petit, Kyra J. E. Borgman, Jérômine Vigneau, Maud Gautier, Sakina Zaidi, Gaëlle Pierron, Sarah Watson, Nadège Gruel, Clémence Hénon, Sophie Postel-Vinay, Marc Deloger, Virginie Raynal, Sylvain Baulande, Karine Laud-Duval, Véronique Hill, Sandrine Grossetête, Florent Dingli, Damarys Loew, Jacob Torrejon, Olivier Ayrault, Martin F. Orth, Thomas G. P. Grünewald, Didier Surdez, Antoine Coulon, Joshua J. Waterfall, Olivier Delattre.
Molecular Cell (2022), https://doi.org/10.1016/j.molcel.2022.04.019
[1] Ewing’s sarcoma is the second most common malignant bone tumor after osteosarcoma in adolescents and young adults. It develops mainly in the pelvic bones, ribs, femurs, fibulae and tibias. Through its high invasiveness, Ewing’s sarcoma can lead to other cancerous sites in the body, including the lungs, skeleton and bone marrow.
[2] Chromosomal rearrangement that involves the reciprocal exchange of chromosomal material between non-homologous chromosomes.
[3] When a single DNA sequence is derived from multiple transcripts or parent sequences.
The management of pediatric cancers at Institut Curie
Every year in France, approximately 2,200 new cases of pediatric cancer are diagnosed. Although more than 80% of children are alive five years after diagnosis, it remains crucial to develop new therapeutic strategies for those who are not yet cured and to reduce the after-effects of conventional treatments. At Institut Curie, between 300 and 400 young patients are treated every year by the multidisciplinary teams at the SIREDO center (Care, Innovation, Research, in Oncology for Children, Adolescents and Young Adults) directed by Dr. Delattre. With the support of the associations, they carry out fundamental, translational and clinical research, with a strong specialization in solid tumors: neuroblastoma, medulloblastoma, Ewing’s sarcoma, retinoblastoma and certain brain tumors.

